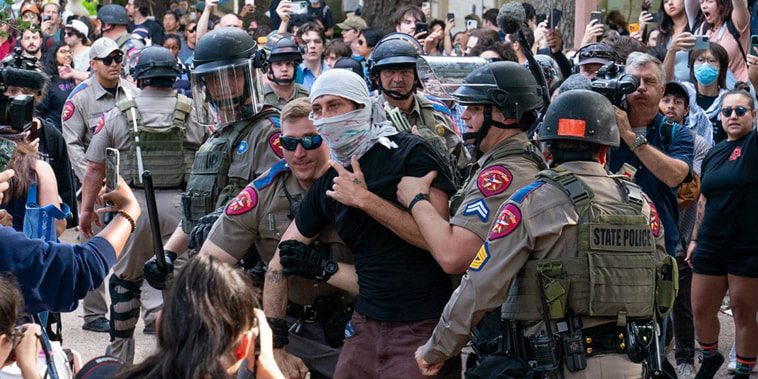TODAY
Police clash with demonstrators as campus protests grow
As a growing number of protests form at college campuses over the ongoing war in the Middle East, some are leading to standoffs with police and are putting university leaders under pressure. NBC’s Stephanie Gosk reports for TODAY.