People suffering from a rare autoimmune disorder that causes their hair to suddenly fall out, often in clumps, now have a treatment option for the first time.
On Monday, the Food and Drug Administration approved baricitinib, a once-a-day pill developed by the drugmaker Eli Lilly to treat alopecia areata, an autoimmune disease that triggers sudden hair loss. The drug was originally approved by the FDA in 2018 to treat rheumatoid arthritis.
The drug — part of a class of medicines known as JAK inhibitors — is the first approved treatment in the United States for the condition, which affects more than 300,000 people in the country each year, according to the FDA.
People with the autoimmune disease can experience hair loss anywhere on their body, including around the scalp, eyebrows and eyelashes, according to the American Academy of Dermatology. The condition can develop at any age, though most people develop it during childhood or during their teenage years, according to the organization.
Alopecia recently gained national attention after actor Will Smith infamously slapped Chris Rock at the Oscars after the comedian made a joke about actor Jada Pinkett Smith, Smith’s wife, who suffers from a different form of the condition than the one treated by Eli Lilly’s drug.
Baricitinib helps regrow hair by preventing the body’s immune system from attacking hair follicles.
Two phase 3 clinical trials, involving a total of 1,200 patients with severe alopecia areata, found that about 40 percent of people who received a daily 4-milligram dose of the drug regrew all or almost all of their hair after 36 weeks.
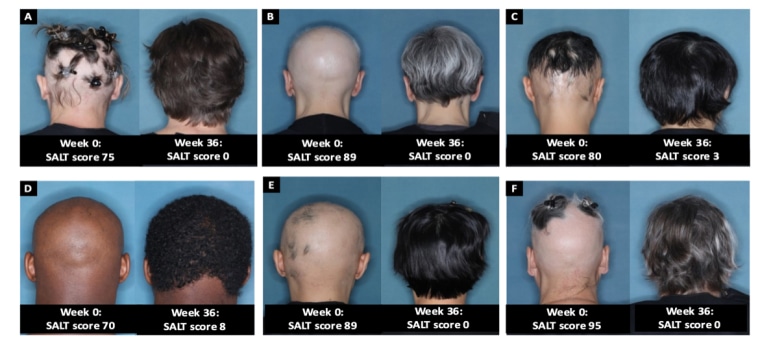
That compares to the about 20 percent of people who received a lower 2-milligram dose of the drug and less than 6 percent of the people in the placebo group.
People in the trials had lost at least 50 percent of hair from their scalp before taking the drug.
The drug is a breakthrough for people with the autoimmune disorder, said Dr. Brett King, a Yale dermatologist who was the principal investigator for the two trials. Many use wigs or scarves to hide their condition.
“It’s a devastating disease,” he said. “Try to imagine you wake up with a spot one day. And then, imagine, three weeks later, or three months later, or three years later, imagine all of your hair going away.”
Dr. Luis Garza, a dermatologist at the Johns Hopkins University School of Medicine who treats people with alopecia, said he’s had patients quit their jobs or avoid going out in public altogether over the stress of losing their hair. He was not involved in the research for the drug.
Up until now, there weren’t many options for people with the condition: They often relied on steroid injections or unproven creams in an attempt to reverse the condition, Garza, who researches hair loss, said.
People can take other drugs that may help, such as JAK inhibitors ruxolitinib or tofacitinib, but they are not approved by the FDA for alopecia, he said. That means they can only be prescribed off-label, which insurers often don’t cover and can cost patients as much as $5,000 a month, he said said.
The out-of-pocket cost for patients can be “insane,” Garza said.
The wholesale price of Eli Lilly’s baricitinib is $2,497.20 for a 30-day supply of 2-milligram pills, Marlo Scott, a spokesperson for Eli Lilly, told NBC News in an email. The company also offers a program that allows people to pay $5 or $25 a month, depending on whether the patient is covered by insurance, she said.
The most common side effects associated with the drug include upper respiratory tract infections, headache, acne and high cholesterol, according to the agency.
The medication may not be for everyone: People with an ongoing infection, cancer or a history of heart attack or stroke should consult with the doctor before considering the medication, Garza said.
King, the principal investigator for the trials, noted that two other similar drugs, from Pfizer and Concert Pharmaceuticals, are also in development to treat the condition and could be approved by the FDA within the next few years.
This story originally appeared on NBCNews.com.