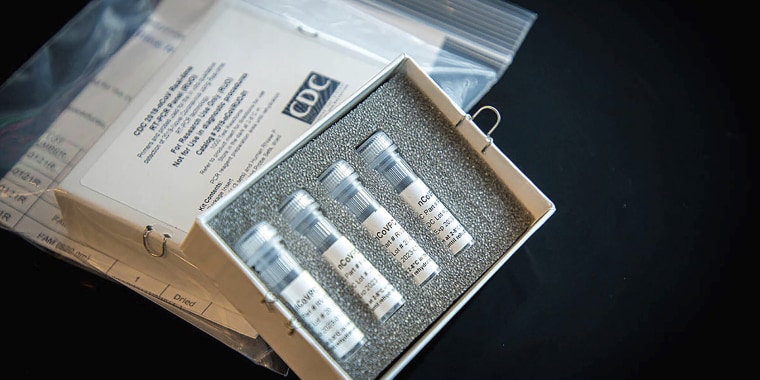
After a weekslong delay, thousands of coronavirus test kits are headed to state and local laboratories, Vice President Mike Pence said Wednesday.
But questions remain about when, exactly, those promised test kits will arrive and how well they will work.
Full coverage of the coronavirus outbreak
"We have more than 2,500 kits that are being distributed around the country this week that will make more than 1.5 million tests available at hospitals that have requested them, and in areas of the country that have been particularly impacted by the coronavirus," Pence said during a meeting Wednesday with diagnostic lab CEOs.
Federal health officials have been scrambling to increase access to coronavirus testing following a series of initial blunders, including limiting testing strictly to those with symptoms and those who had either come into contact with a known patient or had traveled from China.
What's more, the test kits the CDC first sent labs in January proved to be faulty, giving inconclusive results.
Such a large-scale snafu appears to be unprecedented.
"I can't remember it ever happening before," Kelly Wroblewski, director of infectious diseases for the Association of Public Health Laboratories, told NBC News. "This was just, perhaps, the most unfortunate timing to have a manufacturing issue."
On Tuesday, Food and Drug Administration head Dr. Stephen Hahn testified before a Senate committee that the FDA was working with a private company to increase testing capacity, though this will not happen immediately. Wednesday afternoon, Pence said that two of the major commercial diagnostic labs, LabCorp and Quest Diagnostics, would be conducting the test next week.
As of Wednesday morning, only about 70 public health labs were able to test for the coronavirus, Wroblewski said. (The private company mentioned by Hahn will be providing tests to other labs, not public health labs.)
Those 70 include hospitals, military bases, Department of Defense labs, as well as other state and local public health labs, including the Centers for Disease Control and Prevention.
The Association of Public Health Laboratories estimates that if 100 labs are up and running by the end of this week, about 10,000 tests per day could be performed.
"Everybody feels better this week than they did last week," Wroblewski said. "For the last three weeks or so, we've been so focused on having a test up and running. We're excited to be in a place where we're able to do that."
The Department of Forensic Sciences in Washington, D.C., a public health facility, started testing for the new coronavirus on Monday.
"Right now, we're targeting about 25 samples per day. That's what our current capability is," department Director Jenifer Smith said.
She told NBC News the department hopes to have the capacity to process up to 80 samples a day by next week.
"If we need to scale up, we will consider putting more people on the tests or adding additional equipment," Smith added.
The source of the problem with that first round of test kits remains unknown. The CDC said it's investigating.
"Contamination is one explanation, but there are others. And I can't really comment on what is an ongoing investigation," Dr. Nancy Messonnier, head of the CDC's National Center for Immunization and Respiratory Diseases, said during a media briefing Tuesday.
"Our focus is on moving forward," she said, adding the CDC is "very confident" in the new kits.
Neither cost nor diagnostic criteria should deter any American from being tested, Pence said this week.
Speaking to reporters at the White House on Tuesday, Pence said that a doctor's order was all that would be needed for any American to be tested for the novel coronavirus.
"We're issuing new guidance, effective immediately, from the CDC that will make it clear that any clinician or health authority can administer the test," he said.
On Wednesday, he said the test will be covered by private health insurance, as well as Medicare and Medicaid.
CORRECTION (March 5, 2020, 9:31 a.m. EST) : An earlier version of this story erroneously used the incorrect byline, Samantha Kubota. The correct byline is Erika Edwards.