One lot of the Mibelas 24 Fe birth control pills has been voluntarily recalled by Lupin Pharmaceuticals because of a packaging error that puts placebo pills at the beginning of the pack rather than at the end, according to a Food and Drug Administration announcement.
The lot in question is L600518 with an expiration date of May 2018.
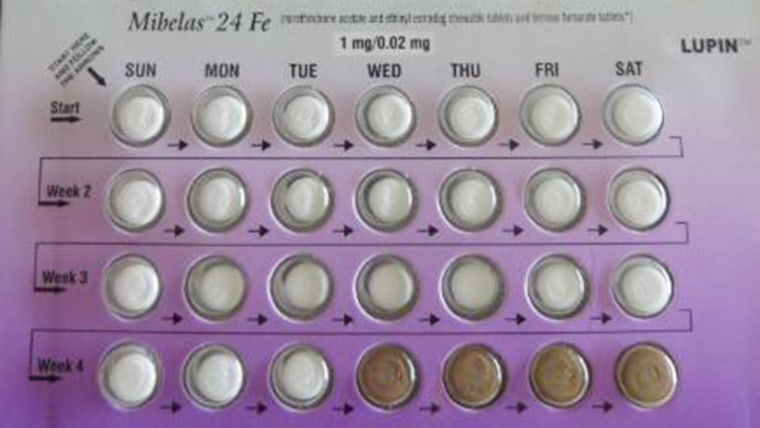
Women who take the pills in the order they appear in the recalled packs are at risk of unintended pregnancy.
And this could have very serious repercussions for some.
“For patients in whom a pregnancy is contraindicated or in whom concomitant medications may have teratogenic effects, an unintended pregnancy may cause significant adverse maternal or fetal health consequences, including death,” the FDA announcement warned.
The risk of unplanned pregnancy associated with these pills is very real, said Dr. Lauren Streicher, an associate professor of obstetrics and gynecology at the Feinberg School of Medicine at Northwestern University and director of the Northwestern Medicine Center for Sexual Medicine and Menopause.
“If you take these first four pills thinking that they are the real thing, you may be off the pill for eight days instead of four and that increases the likelihood of inadvertent pregnancy,” Streicher said, adding that it’s not like missing a single pill in the middle or end of a cycle, which does not increase the risk.
If you’re already in the middle of your cycle with these pills, but haven’t had sex, then your risk isn’t elevated, though you will need to use a back-up means of pregnancy prevention, like condoms, Streicher said.
For those who have already started a pack of the out-of-sequence pills who have had sex, Streicher’s advice is: “Pray. And continue to take the rest of the pack and use back-up contraception for the remainder of the cycle, such as condoms. If they don’t get their period when they finish the pack, do a pregnancy test.”
The FDA recommends: "Consumers with questions regarding this recall can contact Lupin by phone 1-800-399-2561, 8:00 am to 5:00 pm EST, Monday through Friday.